Page Contents
There are many choices available for particle flow visualization in water. The primary requirement is that the particles be 100 microns or less in diameter, and that they not contribute undesirable physics, such as by dissolving or being toxic. Neutrally buoyant particles are desirable but often not required. There are two main categories: solid particles and hydrogen bubbles. We’ll start with solids.
Rheoscopic Fluids
When the particles are oblong, shiny, and densely seeded, you get a rheoscopic fluid — literally ‘current showing.’ The shine depends on fluid motion orienting the particles; it won’t work in a stagnant flow. Exactly how the orientation and flow state relate is complex, limiting this technique to qualitative applications to date. According to mica or aluminum flakes were used for most of the 20th century. However, mica and aluminum are much denser than water, and even 100 micron particles tend to settle out rapidly, limiting their use to relatively high speed flows. Nevertheless, metal-oxide-coated mica flakes are chemically inert, nontoxic, and refractory (don’t burn or melt). They are used extensively in paint pigments and are sold inexpensively in art supply shops and online as beautiful glittery powders. Jaquard Pearl Ex Interference Blue is one of my favorites.
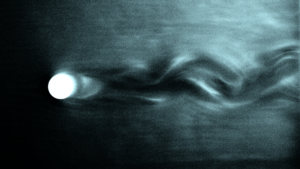
In the mid 1960s Kalliroscope, a fluid developed by artist Paul Matisse, was popular. It contained crystalline guanine — shiny, small (60 microns), with a density not much more than water’s — derived from fish scales. However, it became quite expensive, and production ceased in 2014. Borrero-Echeverry, Crowley and Riddick discovered an inexpensive alternative in 2018 : microscopic stearic acid crystals, which are easily and cheaply extracted from shaving cream. It is not quite as shiny as Kalliroscope, but its density is largely identical to water, and solutions retain their optical properties for years without settling out. One slight disadvantage to be aware of is that the crystals melt at temperatures above ~50 C. Figure 1 shows flow around a circular cylinder using these stearic acid crystals.
Solid Particles for Water
Some water-appropriate particles have already been discussed in the context of particles for air: alumina (which sinks over time), and pine pollen and lycopodium powder (which float on the water surface). Tap water may contain sufficient particulates that no additional particles are needed, especially if laser light sheets are used for illumination.
If neutral buoyancy is required, corn starch powder has a range of densities close to that of water. It is inexpensive and nontoxic, so it can be dumped down the drain without environmental concerns. However, take care to keep concentrations dilute so that particle-particle interactions don’t occur, unless you are really trying to create oobleck with all of its fascinating non-Newtonian behavior .
If a specific density particle is needed, glass or polymer microspheres are available with various diameters and optical properties, but these tend to be costly at $10 to >$200 per gram . You won’t want to dump these down the drain.
Hydrogen Bubbles
In the classic 1963 film Flow Visualization by Stephen J. Kline — part of the National Committee on Fluid Mechanics Films series — flow in a water tunnel is visualized using hydrogen bubbles to illustrate the basics of streamlines, streaklines, timelines, and pathlines (Figure 2). Hydrogen bubbles’ major benefit over markers like a dye is that the bubbles dissolve fairly quickly, so they don’t contaminate a recirculating water channel system or water tank. They also scatter light well, reducing the need for intense lighting. However, like other boundary marking techniques, they are best used in laminar flows.
Figure 2: Flow Visualization by Stephen J. Kline, part of the National Committee on Fluid Mechanics Films series . Copyright 1963, Educational Services Incorporated.
Hydrogen bubbles are formed by electrolysis. A voltage is applied in water between a cathode and an anode; water molecules (H<sub>2</sub>O) split into hydrogen molecules (H<sub>2</sub>) and oxygen molecules (O<sub>2</sub>) as shown in Figure 3. Note that you get twice as much hydrogen as oxygen.
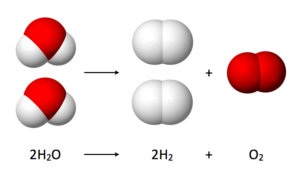
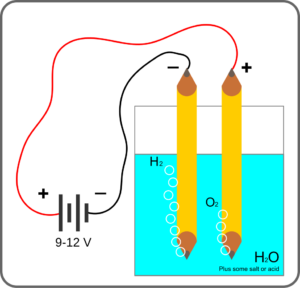
Figure 4 shows a simple demonstration setup using the graphite in pencils as both the cathode and anode, but in flow visualization, a thin wire is used for the negatively-charged cathode, where hydrogen is generated, while a larger, sturdier anode (where oxygen is generated) is placed downstream of the region of interest. Steel wire of 2 mm diameter can be used for the cathode but finer (< 1µm) platinum wire is preferred . Smaller diameter wire is better because the bubble diameters are on the order of the wire diameter, and smaller bubbles will have less buoyancy. Platinum is better than steel; it is less vulnerable to corrosion and ductile enough to be formed into smaller diameters. However, smaller diameter wires are much more fragile, so you’ll have to experiment to find the best combination of parameters.
A 9-volt battery works for a short wire, but a larger setup like the multiple wire rake shown in the video above requires a power supply of up to 300 VDC and several amps of current. The water needs to be conductive; table salt can be added to achieve this, although sodium sulfate is reportedly better . Sodium sulfate is a laxative and is not considered hazardous by the EPA . However, electricity mixed with water can be fatal to humans. Discharging a 9-volt battery through your body won’t damage you (ever licked one?) but even a low voltage (<50 V) with sufficient current (10 mA) can hurt . Dry skin can protect you but wet skin conducts well. Make sure you are not part of the electrical circuit!
A few more points to keep in mind: 1) Any metallic ions in the water are attracted to the electrodes, and essentially electroplate onto them them over time, fouling them. To clean the electrodes, the current must be reversed every once in a while. 2) The cathode wire must be kept smooth and kink free because kinks will make large bubbles. 3) Added salt will enhance bubble formation, but too much will result in overly large bubbles. 4) The chloride in table salt will make a small amount of free chlorine in the water during the electrolysis process — this is handy — it keeps your water disinfected. On the other hand, you won’t want to use the hydrogen bubble technique if studying marine animals.
Electrolytic Precipitation Technique
This method is used to precipitate metals out of a water solution . Similar to the hydrogen bubble technique, it involves electrolysis. In contrast to the hydrogen bubble method, the positively-charged anode is a metal wire or plate, placed where the tracer is desired, and the cathode is downstream. The anode metal dissolves/erodes due to the electrical current and forms a micron-sized solid precipitate . This technique was used to create the famous Karman vortex street image (by Taneda 1977) that graces the cover of Van Dyke’s Album of Fluid Motion . (Too bad it’s copyrighted and I don’t know who to contact for permission to include it here.) One advantage of this technique is that is requires only 10 VDC and 10 mA, and makes a beautiful white ‘smoke’ that follows the flow faithfully. A disadvantage is that the metal precipitates that must be properly disposed of.

