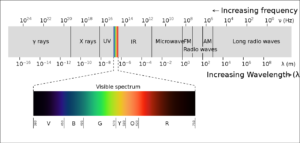
So yes, light is a wave and a particle. I’ve learned to think of a photon as a packet of energy, moving at the speed of light, that has a particular wavelength, frequency and color associated with the amount of energy it carries. A high energy photon has a short wavelength, high frequency, and is at the blue end of the spectrum, and a low energy photon has a longer wavelength, and is at the red end. The color we associate with a photon depends on its energy which is proportional to its frequency: E (energy in joules) = h (Planck’s constant) times frequency ν (hz) . In a given medium (air or water etc.) the frequency ν = speed of light c divided by wavelength λ. In different media the speed of light and the wavelength change, but not the frequency or photon energy, so the color stays the same. We usually talk about color with reference to the wavelength in vaccuum (or air, very close), though.
It’s handy to memorize a couple of visible wavelengths, and I remember them because I’ve worked with certain lasers that I recall fondly. Blue is 4880 Å (angstroms) or 488 nanometers. Green is 5140 Å. All visible wavelengths are on the order of half a micron, which is important to remember because you can get diffraction effects when the wavelength is close to a particle or aperture size. Electromagnetic wavelengths outside of the visible spectrum are harder for me to keep straight so the figure above is a reminder. It’s also helpful to note that wavelengths shorter than the visible spectrum are high energy, and more likely to damage humans; longer wavelengths, not so much.

